PVC degradation is the result of dehydrochlorination reaction in which hydrogen chloride (HCl) is split off. Without PVC stabilizers, free HCl generation accelerates the dehydrochlorination process, resulting in a chain reaction called the Zipper reaction. HCl and conjugated double bond (a single bond between two or more double bonds) are formed, resulting in PVC with altered properties and discoloration that intensifies with increasing conjugation length.
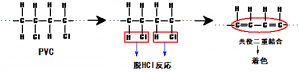
Figure 1: Degradation of PVC
To prevent this phenomenon, metal carboxylates with zinc (Zn), barium (Ba), calcium (Ca), tin (Sn), lead (Pb), and cadmium (Cd) are used. The reaction between metal carboxylates and free HCl inhibits the degradation of PVC (Figure 2). Metal carboxylates can also react with labile allylic chlorine on PVC, which is likely a starting point of the polymer degradation and thereby inhibit the formation of conjugated double bonds in PVC (Figure 3).
![]()
Figure 2. Metal carboxylate [Me(OOCR)2] capturing hydrochloric acid

Figure 3: Inhibition of conjugated double bond formation in PVC by metal carboxylate [Me(OOCR)2]
Although PVC coloration mainly takes place during heat processing, it also occurs when the polymer is exposed to temperatures near 100°C or to ultraviolet light after processing.
In order to mitigate the degradation caused by these various factors, a combination of several raw materials such as antioxidants (phenol and phosphite), early color inhibitors, and UV absorbers must be used to achieve a stabilizing effect tailored to each PVC products and their applications. These chemical additives are known as stabilizers.
Westlake Akishima offers a wide range of custom-made stabilizers suitable for various PVC products. Do not hesitate to contact us if you are looking for PVC stabilizers!
References
FURUYA Masayuki, Vinyl Chloride Resin, Nikkan Kogyo Shimbun, Ltd.
YOSHIDA Tokiyuki, SHINDO Shinichi, OGAKI Tadayoshi, IDE Kesaichi, Properties and Applications of Metallic Soaps, Saiwai Shobo
